 magnify / A nurse offers a boy a dose of the Pfizer vaccine at a COVID-19 vaccine sanatorium in Florida on the day before classes begin for the 2021-22 faculty year. Getty | Paul Hennessy reader comments 2 with 2 posters taking part Share this story
magnify / A nurse offers a boy a dose of the Pfizer vaccine at a COVID-19 vaccine sanatorium in Florida on the day before classes begin for the 2021-22 faculty year. Getty | Paul Hennessy reader comments 2 with 2 posters taking part Share this story A panel of independent scientific specialists advising the meals and Drug Administration voted 17 to 0 (with one abstention) this afternoon in choose of authorizing the Pfizer-BioNTech COVID-19 vaccine for use in toddlers ages 5 to 11 years old.
The vote is a key step toward the primary pediatric COVID-19 vaccine within the US. subsequent, the FDA will should log off on the recommendation from the advisory panel—the Vaccines and related organic items Advisory Committee (VRBPAC)—and situation an emergency use authorization for the 5-to-eleven age community. That FDA's authorization is anticipated within days. as soon as that occurs, the federal executive will begin delivery pediatric doses of the vaccine to states for distribution at clinics, pharmacies, and pediatricians' workplaces.
however before doses can go into any little arms, a panel of independent experts for the centers for disease handle and Prevention will additionally need to weigh in. That panel—the Advisory Committee on Immunization Practices (ACIP)—is scheduled to satisfy November 2 and 3. If ACIP votes in choose of recommending use of the vaccine in infants a long time 5 to eleven, CDC Director Rochelle Walensky will need to sign off on the committee's suggestion. that could likely happen rapidly, and after that, vaccinations can start.
Many folks and caretakers are anxiously anticipating the vaccine's availability. With kids again in colleges for in-adult studying and fall and wintry weather vacations looming, there is clear eagerness to have a protecting vaccine for school-aged infants. besides the fact that children, there is additionally a variety of challenge and anxiousness amongst some about offering vaccines to those youngsters, who have lessen risks than adults of developing extreme COVID-19. one of the crucial largest concerns is vaccine-linked myocarditis (irritation of the coronary heart), which has proven up as a big risk for older young adults, specifically adult males a long time sixteen and 17. This all makes weighing the dangers and advantages of vaccines in the 5-to-eleven community especially difficult.
whereas modern-day favorable vote changed into largely expected, VRBPAC's all-day assembly Tuesday became no cakewalk. Committee individuals carefully combed via efficacy, defense, and modeling records in advance of their vote. And there turned into plenty of disagreement and hesitation. simply prior to the vote, some committee members floated the concept of best authorizing the vaccine for high-possibility children. in the end, the committee decided that the efficacy and security facts turned into sufficient to subject an authorization, though issues lingered about possible mandates.
Vaccine recordsprevious to brand new assembly, Pfizer had released facts indicating that smaller doses of its COVID-19 vaccine might safely and robustly protect little ones. preliminary information advised that two shots of just a 3rd of the grownup dose—10 micrograms as an alternative of the grownup dose of 30 micrograms—produced related antibody stages in children a long time 5 to eleven as those considered in vaccinated teens and younger adults. The smaller doses in younger children also perceived to have an identical facet results as viewed in older organizations, similar to fatigue, headache, fever, and chills.
commercialmoreover, a trial run via Pfizer and BioNTech involving about 2,250 little ones counseled that the pediatric formula was about 91 % helpful at preventing symptomatic disorder. The trial ran during the delta wave, suggesting that the vaccine is positive towards the particularly transmissible variant.
ultimate week, the FDA launched its interior review of the statistics showing that the vaccine's benefits of thwarting the pandemic coronavirus and fighting COVID-19 circumstances would "obviously outweigh" the dangers of myocarditis.
In opening remarks at state-of-the-art assembly, Peter Marks, director of the FDA's center for Biologics contrast and research, unambiguously brought up the case in prefer of authorizing the vaccine.
"far from being spared from this hurt of COVID-19 within the 5 to eleven 12 months historic age range, there have been over 1.9 million infections, over 8,300 hospitalizations, about a 3rd of which required intensive care instruments stays, and over 2,500 circumstances of multisystem inflammatory ailment from COVID-19," Dr. Marks noted. "And there have also been close to 100 deaths, making it some of the precise 10 factors of dying during this age latitude all over this time. moreover, infections have brought about many faculty closures and disrupted the education and socialization of little ones."
COVID hazardsadditional facts introduced by the CDC confirmed that infants 5 to 11 are making up a larger share of COVID-19 cases. The children accounted for 10.6 p.c of all cases as of prior this month, regardless of being only eight.7 p.c of the population. and that's the reason doubtless a big undercount. Antibody screening means that around forty two percent of infants during this age latitude were infected.
notwithstanding 5- to 11-12 months-historical children have decrease fees of hospitalization than other pediatric businesses, there have been eight,300 hospitalizations within the age community. Black, Hispanic, and American Indian/Alaska Native little ones had the highest charges of hospitalizations within the cohort. in one facts set, 68 p.c of hospitalized 5- to eleven-year-historic infants had at least one underlying medical situation, equivalent to asthma or weight problems. however 32 p.c had no underlying circumstances.
MyocarditisThe committee additionally heard detailed facts on the chance of myocarditis, which is most gigantic in younger males. however the records we've so far suggests the risk may also peak in adult males a while 16 to 17, comparable to what's viewed in "basic" myocarditis instances from the prepandemic era. for example, in line with the latest records, there were round 70 cases of myocarditis for every 1 million doses of vaccine given to males a long time sixteen to 17. however the charges dropped to about 40 instances per 1 million doses in males 12 to 15 and 37 circumstances per 1 million doses in males 18 to 24.
This mirrors what's been seen earlier than with myocarditis. Case rates standard are higher in males, and the chance is bimodal with age. babies under 1 12 months old and youths sixteen to 18 have the maximum rates of hospitalization for myocarditis. Between infancy and adolescence, the possibility is low, and it declines step by step after the adolescent top. Why this vogue exists is uncertain, however scientific consultants suspect that the newborn top in myocarditis is linked to congenital complications and the adolescent height is regarding testosterone ranges—which also helps clarify why or not it's above all considered in adult males.
advertisement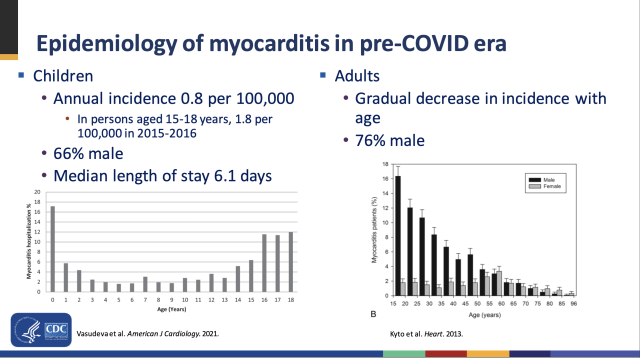 magnify / Myocarditis risks by age and sex.
magnify / Myocarditis risks by age and sex. The similarities between the pattern rising from vaccine-connected myocarditis and "classic" myocarditis make scientific consultants hopeful that 5- to eleven-12 months-olds may have tons lessen dangers of myocarditis than are viewed in the 12-to-15 and 16-to-17 age businesses. an extra high quality aspect: basically all instances of vaccine-linked myocarditis continue to be frequently mild, and most kids improve fully.
but with none data on the specific 5-to-eleven age neighborhood, it be impossible to assess the youngsters' specific chance. Even within the maximum-possibility neighborhood so far—male teens 16 to 17—the rates of myocarditis are too small to determine devoid of millions of individuals getting vaccinated.
Modelingin the absence of fulfilling statistics, the CDC became to thorough chance/improvement modeling to explore all of the feasible effects. The company modeled the dangers and benefits of vaccinating 5- to eleven-yr-olds in a variety of eventualities, beginning with COVID-19 case charges at levels seen in early September, assuming vaccine efficacy become 70 percent towards instances and eighty % against hospitalizations, and assuming the fees of myocarditis were the equal as these considered in kids a long time 12 to fifteen (situation 1). Researchers then tweaked the mannequin outcomes if COVID-19 case prices were bigger or lower (scenarios 2 and 3, respectively), if vaccine efficacy changed into greater (situation 4), if COVID-19 demise rates have been better (state of affairs 5) and if costs of myocarditis instances had been decrease (state of affairs 6).
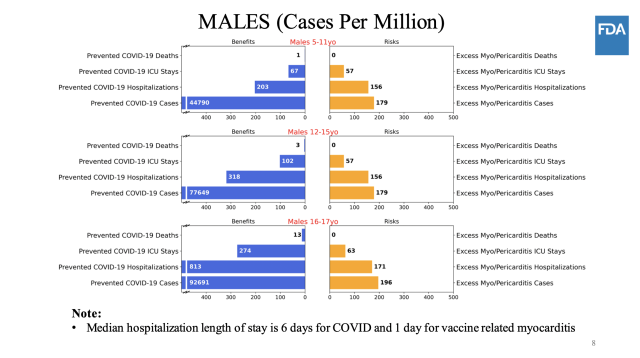 enlarge / scenario 1
enlarge / scenario 1 In all of the eventualities—except when COVID-19 case costs had been very low (scenario three)—the benefits of vaccinating children 5 to 11 outweighed the risks of myocarditis. In state of affairs 3, the mannequin predicted hospitalizations and ICU stays from vaccine-connected myocarditis would outnumber the COVID-19 hospitalizations and ICU that vaccines would prevent. still, as a few consultants referred to in the discussion intervals, the myocarditis rates utilized in all via situation 6 are doubtless big over-estimates.
With all the uncertainty and complex modeling statistics, was clear hesitation and a lack of enthusiasm for authorizing the vaccine for all children amongst one of the most VRBPAC individuals . Some advocated for authorizing the vaccine just for toddlers at high chance of severe ailment, comparable to those with bronchial asthma or obesity. "It simply appears to me that in some ways we're vaccinating little ones to protect the adults, when it's going to be the other way around," spoke of James Hildreth, voting VRBPAC member and president of Meharry clinical college. "So, this is a really challenging one for me, however I do agree with that little ones at optimum chance do should be vaccinated"
Others openly worried that the authorization would lead to vaccine mandates for all children earlier than extra protection records may accumulate to enhanced determine the chance/improvement calculations. "I consider [mandates] could be an error at this time until we get more information concerning the protection," referred to Cody Meissner, vote casting VRBPAC member and pediatric infectious disease professional at Tufts scientific middle.
previous this month, California Gov. Gavin Newsom introduced that the state will add COVID-19 vaccines to the list of required immunizations for student— but most effective once the FDA promises a full approval. modern-day vote become most effective on an emergency use authorization, now not full approval. The FDA's Peter Marks noted that states and officials have tended to hesitate on issuing mandates ahead of full FDA approval. moreover, Marks also stated a couple of times that such issues of mandates and specifics of use had been out of the scope of VRBPAC's purview. here is more the realm of ACIP and public health officers.
commercial "toddlers are loss of life"CDC respectable Dr. Amanda Cohn chimed in to convey the dialogue again to advantages and hazards when it comes to little ones, for which we (as a society) are likely to settle for few hazards. "it is pretty clear to me that the benefits do outweigh the chance when I hear about toddlers who're being put in the ICU, who are having future consequences after their COVID—and youngsters are loss of life," Cohn talked about. "We vaccinate mechanically against a couple of vaccine-preventable ailments for which a long way fewer deaths and hospitalizations and ICU admissions take place." although she went on to acknowledge the possibility of myocarditis, she noted that fees are probably reduce than the older teenagers and the decrease dose of the pediatric formula may probably reduce the possibility additional. furthermore, the CDC has strong safeguard methods deploy for monitor for dangers relocating forward.
common, the committee felt the benefits did outweigh the hazards, regardless of probably the most hesitations. Hildreth, who ended up balloting sure, cited after the vote that he was swayed to authorization because of availability issues. "I are looking to be certain the babies who actually need this vaccine—primarily the black and brown infants in our country—get this vaccine," he talked about.
Now the decision is to the FDA to situation the authorization. If it does, the challenge will movement to the CDC for use ideas.


0 Comments